Biology concepts – evolution, reproductive advantage,
natural selection, co-dominance, X-linked genes
Last week we learned how less aggressive strains of malaria were used to treat neurosyphilis and how they may be useful in treating HIV
infection. This week, we will turn 180˚ and see if other diseases can help
prevent or lessen the effects of malaria. In the process, much can be learned
about natural selection and reproductive advantage.
As you
undoubtedly remember from last week, malaria is a parasite-caused infectious disease that is
transmitted from human to human by mosquitoes. The parasite, Plasmodium falciparum, takes up
residence in the red blood cells (RBC) to reproduce. The red cells burst to
release the organisms, and this brings fever and weakness.
As far back as the 15th and 16th
centuries, quinine, made from the
bark of the cinchona tree, was being used in Peru to treat malaria. Chloroquine,
mefloquine, and quinine all work against malaria in similar fashion. Because of
their neutral pH, they move across membranes easily including the lysosome
membrane. Once inside the lysosome, they become charged and can’t get out. This
includes the trophozoite-containing lysosomes. In the RBC, trophozoites consume
hemoglobin to obtain amino acids, and the heme is digested in the lysosomes to
form a black malaria pigment. The quinine drugs in the lysosome bind up the
heme and produce a toxic product (cytotoxic heme) that kills the parasite.
There are other classes of drugs that are useful against P. falciparum. Primaquine and the
artemisinin drug, artesunate, act by a completely different mechanism from that the
quinine drugs. Artesunate is excellent for treating P. falciparum malaria, while primaquine is often used in
conjunction with quinine to treat P.
vivax or P. ovale forms of the
disease.
These drugs work by breaking down – weird, but this is how
many drugs work. It isn’t what you swallow that kills the organism, it's the metabolites (the products made by your
biochemistry breaking down the drug) that are active. In the case of artesunate
and primaquine, the heme molecule in the red blood cells releases peroxide from
the parent compound (the drug you take). This is just like the peroxide you use
to wipe out cut in order to prevent infection.
The cell has defenses against free radical damage, but
higher than normal concentrations render the RBC fragile; on the tipping point
of destruction. Treatment with primaquine or artesunate makes the cell
inhospitable for the parasite, the red blood cells become flop houses instead
of five star hotels. The parasite’s operating instructions are to survive and
reproduce, but these drugs pull up the erythrocyte welcome mat and the parasite
seeks moves on to seek friendlier accommodations.
Unfortunately, some strains of P. falciparum have become resistant to some quinine drugs,
especially chloroquine. The free radical generating drugs are still useful, but
scientists in Western Cambodia recently reported artesunate drug resistance
there. The parasite has evolved – evolutionary pressure is everywhere. The
actions of humans have put pressure on the organism to evolve; those
parasites with mutations to resist the drugs have a reproductive advantage, and
those mutations get passed on. We had better have something else on our plate to
combat malaria – we're working on it, but nature has provided some help as well.
There are natural defenses against malaria. We have seen
that a fragile red blood cell helps in preventing are lessening the disease
course of malaria. What else might do that? This is where human genes come into
play.
Sickle cell disease
creates a very fragile RBC. The mutation is just a single DNA base change in the
hemoglobin beta chain peptide, but the result is a hemoglobin molecule that
becomes pointy and can tear the red blood cell apart, or can get stuck in small
blood vessels and prevent good blood flow. Reduced blood flow starves the
downstream tissues of oxygen.
You get one gene for hemoglobin beta chain from each parent.
The disease comes when an individual receives mutated genes from both parents.
But that doesn’t mean that sickle cell anemia is a recessive trait. If you have
one copy of the mutated gene, then you will have sickling problems when oxygen
concentrations are low, like during exercise or at high altitude.
If sickle cell anemia was a recessive disease, then a single wild type (normal) gene would be dominant, and you would show no disease. Instead, sickle cell
anemia is co-dominant, one mutated
allele (copy of the gene) is like having half the disease; it only shows up in
certain circumstances.
This can still be a pebble in your shoe, just ask Ryan Clark, the Pro-Bowl safety
for the Pittsburgh Steelers. In a 2007 game in Denver (altitude 5300
ft, 1616 m), Ryan almost died from a sickling attack during the game, and ended
up having his spleen and gall bladder removed (remember that sickled RBCs can clog
blood vessels, especially in blood rich organs like the spleen).
When Pittsburgh next played Denver, Clark didn’t even make
the trip. This just happened to be the 2011 playoff game in which Tim Tebow
threw a long touchdown pass in overtime to the receiver being covered by Clark’s
replacement. Sometimes disease can change how sports evolve as well.
Thalassemia is
another example. This is a group of inherited disorders wherein there is reduced
production of one of the subunits of hemoglobin (hemoglobin is made from 2
alpha and 2 beta subunits). Alpha-thalassemias have mutations in the alpha
subunit; likewise for beta-thalassemia.
Reduced subunit number means reduced hemoglobin number; the
blood won’t carry enough oxygen, and the patient is constantly oxygen-poor in
his/her tissues. Having two mutated alpha genes is lethal in the very young
(called hydrops fetalis), but you can live with one mutated alpha gene, one
mutated beta gene, or even two mutated beta genes.
 |
This
the broad bean, or fava bean in opened pod
and
out of the pod in a bowl. The ancient Greeks
used
to vote with fava beans, a young white bean
meant
yes, and old black one meant no.
|
Favism, better
called glucose-6 phosphate dehydrogenase
deficiency (G6PDH), is an X-linked genetic disease; the gene is on the X
chromosome. A female (XX) has two copies, so having one mutant copy is no
problem, but a male (XY) has only one, so getting a mutated copy from your
mother means that you ONLY have the mutated gene – this brings the disease.
The enzyme G6PDH works in several pathways; in your red
blood cells, it is the only source of reduced glutathione, an important
antioxidant. This means that things that trigger free radical formation in your
red blood cells will trigger the disease – lots of weakness and lack of energy.
If there is enough erythrocyte destruction, one could die.
Triggers include broad beans (fava beans), hence the name
favism. Other triggers include many drugs, including primaquine and artesunate,
the anti-malaria drugs that induce free radicals. Having G6PDH-deficiency is
like having your own artesunate pharmacy right in your cells - you naturally have higher oxygen radical levels in your RBCs, so the malarial parasite can't live there.
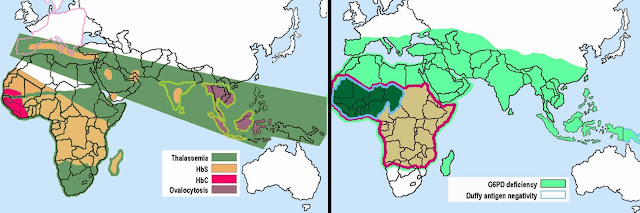
Not by accident, sickle cell mutation is more prevalent in
people of Sub-Saharan African descent, thalassemia mutation is more common in
people from the warm, moist Mediterranean, and G6PDH-deficiency is found most
commonly in the Mediterranean and Southeast Asia. These just happen to be the
areas where malaria-carrying mosquitoes are most abundant. Evolutionary
biologists make the argument that natural selection has maintained these genes
in the populations because they provide a reproductive advantage to the
species.
You
might die from sickle cell disease, but probably not from sickle cell trait or
beta-thalassemia. Learning not to eat fava beans makes the G6PDH mutation less
lethal. One might very well live to an age where one could mate and pass on
his/her genes. The diseases might still kill the patient, just not as soon as malaria would.
Malaria is a killer, and significantly, a killer of the
young. In East Africa, children are bitten by the anopheles mosquito on average
50-80 times each month. They very well might not reach an age to reproduce.
Therefore, having sickle cell trait, thalassemia, or favism provides a
reproductive advantage in these environments and natural selection has resulted
in these alleles remaining in the populations in these areas.
Since
P. vivax uses Duffy Ag as a way to enter
the red blood cells, those with the Duffy SNP are resistant to P. vivax malaria – they don’t even have
to suffer with some other disease; just a simple case of chance. And the prepared mind exploits chance –
the Duffy antigen binding protein is now a candidate for use as a P. vivax vaccine.
Next week, how the plague was defeated by a genetic disease.
Chootong P, Panichakul T, Permmongkol C, Barnes SJ, Udomsangpetch R, et al. (2012). Characterization of Inhibitory Anti-Duffy Binding Protein II Immunity: Approach to Plasmodium vivax Vaccine Development in Thailand. PLoS ONE , 7 (4) DOI: 10.1371/journal.pone.0035769
For
more information or classroom activities, see:
Malaria
–
sickle
cell mutation –
thalassemia
–
favism
–
duffy
antigen –





Thanks for the great giveaway.Wow, what an amazing giveaway!! Thanks so much.
ReplyDelete