Biology concepts – gravitropism, plastid, chloroplast,
chromoplast, amyloplast, leucoplast, malaria parasite
Believe it or not, the way plant roots know to grow into the
dirt is related to photosynthesis! “How can this be?” you ask. Well, let’s talk
about it.
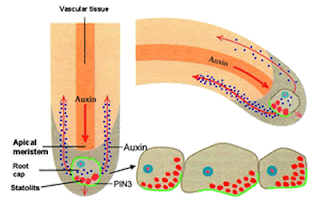
The cells in the tips of the plant rootlet respond
positively to gravity, called gravitropism
(the older word for it is geotropism). If you lay a growing plant on its side,
the roots will respond by growing (turning) toward the gravity within 10
minutes. The mechanism for this stimulation involves tension and a plant
hormone called auxin.
Since the statoliths are connected to the membrane of the
cell by the cytoskeletal actin molecules, so when they settle toward gravity,
some cells in the membrane are stretched and some are compressed. This tension
signals the cells to change the number of receptors for the growth control
hormone auxin. More tension (more
stretch) causes the auxin to move away, toward cells that are under less
tension. Auxin prevents cell enlargement and cell division, so those root tip
cells on the bottom receive more inhibition. Those on top enlarge more and
divide more, so the root turns down. If the root is already vertical, the
tension is equal in all directions, and the growth is equal in all directions –
the root gets thicker and longer.
Gravitropism is related to photosynthesis in that both
mechanisms involve chloroplasts, sort of. Root cells don’t perform
photosynthesis, they are underground, so they don’t have chloroplasts. But they
do have the amyloplastid statoliths, and these are related to chloroplasts.
Both amyloplasts and chloroplasts are specialized versions of the
plant organelle called the plastid.
We asked last week about what defines a plant cell – maybe the plastid is it.
All plant cells have some plastids, but in different plant cells they may take
different forms, including chloroplasts, chromoplasts, leucoplasts, amyloplasts,
elaioplasts, or proteinoplasts, but they all start out as proplastids (pro = early and plastos = form in Greek).
Proplastids become etioplasts,
chloroplasts or leucoplasts. The etioplast is a sort of pre-chloroplast; a
chloroplast without chlorophyll. It is waiting to be stimulated by light energy
before it decides to spend all the energy it requires to make the chlorophyll.
The old science fair project about growing bean plants in the dark demonstrates
the etioplasts. The plants are white when grown in the dark, but bring them
into the light and they soon green up. The sunlight stimulates the etioplasts to
make chlorophyll, become full-fledged chloroplasts and start photosynthesizing.
 |
This
is a photomicrograph of the plastids of a
red
flower petal. The chromoplasts hold the
xanthocyanin
pigments, but we see it as a
continuous
color because they are so small.
|
If the proplastid does not differentiate toward a
chloroplast pathway (etioplast too) then it will become a leucoplast (leuko = white). The leucoplasts don’t have color; they
become specialized for the storage of plant materials. If they store starch,
they are called amyloplasts. Lipid storing leucoplasts are called elaioplasts,
while protein storing plastids are called proteinoplasts. Each type serves a
crucial purpose in the cells they inhabit, and they can all interchange,
depending on the conditions the plant cell finds itself in.
Even more important, leucoplasts that are not serving as
storage organelles have biosynthetic functions. They work in the production of
fatty acids and amino acids. Amino acids link together to from proteins, so
their synthesis is very important for plants. Plants must manufacture every
amino acid it needs, whereas we get many of ours in our diet. There are even some
amino acids that humans can’t make, called the essential amino acids. Of the
twenty common amino acids, nine of them must be taken in through our diet, and
some people with pathologies can’t make up to seven more. Plants don’t have
this luxury; all their amino acids must be made on site. Good thing they have
leucoplasts.
There is one other type of plastid that we haven’t talked
about, the one that is important for the Autumn tourist trade. Etioplasts and
chloroplasts can differentiate into chromoplasts,
organelles that store pigments (colored molecules) other than chlorophyll.
Chlorophyll provides energy through photosynthesis, but they also have a cost.
The old saying, “It takes money to make money” applies to plants as well. It
takes energy to make chlorophyll, so it only pays to make chlorophyll when
there is ample sunlight to put through photosynthesis. When the days get
shorter, the profit margin for producing chlorophyll goes down, so the plant
just stops making it.
 |
Twin
females were imaged after a lifetime of smoking
or non-smoking.
Can you guess who was
exposed to the oxygen radicals in cigarette
smoke
her whole adult life?
|
So the chloroplasts lose their chlorophyll in autumn and
could be called leucoplasts, but the chromoplasts still have the pigments that
had been masked by the greater number of chlorophyll molecules. The trees turn
magnificent colors and bring people from the cities to stay in bed and
breakfasts, and to purchase handmade scarves and way too much maple syrup and
apple butter. Economy and biology are so often interrelated.
Plastids are the quintescential plant organelles – no plant
cell is without them in some form (well O.K., there is one exception, we’ll
talk about that next week). But that still doesn’t mean that they define a
plant cell. Remember that algae are not plants, but they have chloroplasts, and
chloroplasts are one type of plastid. There is even a bigger exception in this
area; some of the apicomplexans.
Certain protozoal organisms, including the malaria parasite
(Plasmodium falciparum) contain an
organelle called an apicoplast. P.
facliparum or its ancestor obtained an algae cell by secondary endosymbiosis (the
primary endosymbiotic event was the algae taking in a cyanobacterium), so the
apicoplast has a four, not two, membrane system.
There is evidence that the apicoplast works in fatty acid and heme synthesis, like the leucoplast or in the production of
ubiquinones that are important for the electron transfer chain in the
mitochondria. There is also evidence that it is involved in FeS cluster
production, like the hydrogenosome
and mitosome. Both
of these pieces of evidence show the interelationships of the endosymbiosed
organelles and the connection between energy production and energy use. Whatever
their functions are, if you destroy or inhibit it the malaria bug dies. As
such, it has been a popular target for anti-malarial drugs.
Malaria parasites cured of their apicoplasts
(cured means freed of) do not die right away. They just can’t invade any new cells and
therefore can’t complete their life cycle. This is why anti-apicoplast drugs
may be a boon to malaria treatment. The biosynthetic pathways in the apicoplast
are the targets of four recent drugs, but the primary way to stop malaria remains
the mosquito net. There is strong hope that a new vaccine, called RTS,S is a
light at the end of the tunnel for this killer of millions.
One final thought on the plastid – an addition to the exception of melanosomes. We discussed a few weeks ago that melanosomes were the only organelles that could move from cell to cell. Well, that isn’t exactly so. I held off on adding the plastid to that list until we had discussed what a plastid was.
A 2012 study at Rutgers University tested whether plastids
and mitochondria could move between plant cells. There results showed that
entire plastid genomes could be seen in recipient cells, and the fact that the
whole chromosome passed indicated that the plastid was probably moving from
cell to cell intact. But there was no movement of the mitochondria, so it is a
plastid (and melanosome) specific event.
The researchers hypothesize that this may be a way for plant cells to
repopulate damaged cells with working organelles. As such, it would be similar
to how mammalian stem cells can move mitochondria into damaged cells during
tissue repair. But that is another story.
We have repeatedly talked about how the mitochondrion and
plastid can replicate on their own and then are portioned out to the daughter cells
when a parent divides. Can it really be that simple? I’ll bet there is a
definite mechanism, and I bet that mechanism has exceptions. Let’s look into
this next time.
Gregory Thyssena,Zora Svaba, and Pal Maligaa (2012). Cell-to-cell movement of plastids in plants Proc Natl Acad Sci U S A. , 109 (7) DOI: 10.1073/pnas.1114297109
For
more information or classroom activties on plastids, gravitropism, or Plasmodium falciparum see:
Plastids
–
Gravitropism
–
207.62.235.67/case/biol215/docs/roots_gravity.pdf
Plasmodium falciparum –